sulfate ion lewis structure|How to Draw the Lewis Structure for the Sulfate Ion : Manila 6K. 976K views 10 years ago Lewis Structures Practice Problems with Answers. A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion). For the SO4 2-. Play at Luck online casino and enjoy a bonus of ⭐Up to £100 ⭐125 Free Spins Play the most popular online slots. Claim your welcome bonus today! . Sign in quick and easy. 100% secure and fast payments. Support 7-days a week, from 6:00 to 22:00 GMT. 1300+ games from biggest game providers. Payment processors.
PH0 · Sulfate
PH1 · SO42 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
PH2 · SO42 Lewis Structure, Molecular Geometry,
PH3 · SO42
PH4 · SO4 2
PH5 · Lewis Structure for SO4 2
PH6 · How to Draw the Lewis Structure for the Sulfate Ion
PH7 · How to Draw the Lewis Dot Structure for SO4 2
PH8 · How To Draw The Lewis Structure of SO4 2
Inabutan ng Ulan Habang Sesex sa Gubat (Pinay Picking Up Wild Rattan Fruit) 1 year. 9:29. Wild Sexy Pinay Gets Fucked and Creampied By Her Driver At The Dining Table 2 years. . KANTOT SA MISIS KONG MALIBOG HABANG NANUNUOD NG JAPANESE PORN MOVIE -PINAY VIRAL SCANDAL 3 years. 8:16. Kainpepe bago pinasok ang .
sulfate ion lewis structure*******6K. 976K views 10 years ago Lewis Structures Practice Problems with Answers. A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion). For the SO4 2-.
Learn how to draw the Lewis structure, molecular geometry, and hybridization of sulfate ion (SO42-) using the VSEPR model and the octet rule. Find out the formal charge, bond angles, and polarity of .
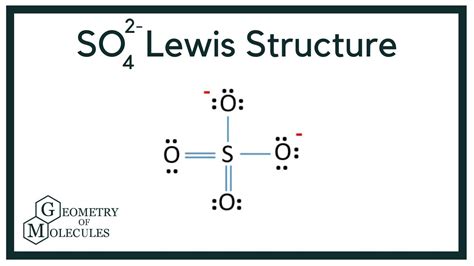
The Organic Chemistry Tutor. 7.75M subscribers. 1.4K. 126K views 3 years ago New AP & General Chemistry Video Playlist. This chemistry video explains how to draw the lewis structure of the. Wayne Breslyn. 767K subscribers. 714. 78K views 3 years ago. A step-by-step explanation of how to draw the Sulfate Ion Lewis Dot Structure (SO42- ). We'll also look at the molecular.What is the SO₄²⁻ ion, and why is its Lewis structure significant? SO₄²⁻ is the sulfate ion, composed of one sulfur atom and four oxygen atoms. Its Lewis structure offers valuable .Lewis Structure for SO4 2- (Sulfate Ion) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and .

See the Big List of Lewis Structures. Transcript: Hi, this is Dr. B. Let's do the SO4 2- Lewis structure, for the sulfate ion. On the periodic table: Sulfur, 6 valence electrons; Oxygen .sulfate ion lewis structure (Sulfate Ion) Watch on. 0:00 / 2:05. In this blog post, we will go through all the details related to this molecule. Right from valence electrons to shape, you will find everything related to SO42- ion here. .Structure. The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry of the isolated anion is the same as that of methane. The .How to Draw Lewis Structures in 5 Easy Steps. Gregory Hodgkins (YouTube) Step 1. Count all the valence electrons for each atom. Add or subtract electrons if the structure is an anion or cation, respectively. Example. SO42-. Sulfate is a polyatomic ion with 1 sulfur (6 valence electrons), 4 oxygens (4 x 6 valence electrons = 24 e -) and a charge .
Hence there are a total of 32 valence electrons for the Sulfate ion. SO42- Lewis Structure. The Lewis Structure of any molecule helps to understand the bonding of atoms in the structure. Apart from that, it also . Lewis structure of SO4 2- ion (Sulfate ion) contains two double bonds and two single bonds between the Sulfur (S) atom and Oxygen (O) atoms. The Sulfur atom (S) is at the center and it is surrounded by 4 Oxygen atoms (O). . In order to draw the lewis structure of SO4 2-ion, first of all you have to find the total number of valence electrons .SO 4 2-.Lewis Structure (Sulfate ion). Lewis structure of sulfate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of SO 4 2-.In lewis structure of sulfate ion, there should be charges on several atoms due to .sulfate ion lewis structure How to Draw the Lewis Structure for the Sulfate IonLewis Dot of the Sulfate Ion. SO 42-. Back. 70 More Lewis Dot Structures. S does not follow the octet rule. It will hold more than 8 electrons. Sulfur having valence electrons in the 3rd energy level, will also have access to the 3d sublevel, thus allowing for more than 8 electrons. Elements in the first 2 periods of the Periodic Table do not . The following procedure will give you the correct Lewis structure for any molecule or polyatomic ion that has one central atom. Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for .The sulfate or sulphate ion is a polyatomic anion with the empirical formula SO 2 . The apparent contradiction can be cleared if one realizes that the covalent double bonds in the Lewis structure in reality represent bonds that are strongly polarized by more than 90% towards the oxygen atom. Oxidation-Reduction: Sulfate is a very weak oxidizing agent. Since sulfur is in its maximum oxidation number in sulfate ion, this ion cannot act as a reducing agent. This page titled Sulfate Ion (SO₄²⁻) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by James P. Birk. Sulfate ion is a very weak base.How to Draw the Lewis Structure for the Sulfate IonGet the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
Drawing an acceptable resonance structure for the sulfate anion (without a lot of math or memorization)!Resonance in the Lewis structure of the sulfate ion refers to the phenomenon where multiple valid structures can be drawn to represent the bonding in the molecule. In the case of the sulfate ion (SO 4 2-), the sulfur atom is bonded to four oxygen atoms. However, the position of the double bonds can vary. The SO42- Lewis structure depicts the molecular arrangement of sulfate, which consists of one sulfur atom and four oxygen atoms. The structure has two double bonds and two single bonds .Resonance structures are used when one Lewis structure for a single molecule cannot fully describe the bonding that takes place between neighboring atoms relative to the . for Sulfate (SO 4 2-). There isn't a most favorable resonance of the Sulfate ion because they are all identical in charge and there is no change in Electronegativity .
How to Draw the Lewis Dot Structure for SO4 2- (Sulfate ion) Optimizing the Structure. To optimize the Lewis structure, we can rearrange the lone pairs of electrons to minimize formal charges and maximize stability. In the case of SO42-, there is no need for further optimization since all atoms have a formal charge of 0, and the octets . The strength of ionic bonding depends on the magnitude of the charges and the sizes of the ions. 10.3: Lewis Structures of Ionic Compounds- Electrons Transferred is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The tendency to form species that have eight electrons in the valence shell is .
Sulfate has a charge of 2 −, which means it has an additional 2 electrons. When drawing the Lewis structure for sulfate, sulfur is placed in the center of the Lewis structure because it is the .Identify the number of valence electrons in each atom in the NH 4 + ion. Use the Lewis electron structure of NH 4 + to identify the number of bonding and nonbonding electrons associated with each atom and then use Equation 4.4.1 to calculate the formal charge on each atom. Solution: The Lewis electron structure for the NH 4 + ion is as follows:The Lewis structure of SO3 2- represents the arrangement of atoms and valence electrons in the sulfite ion. It shows one sulfur (S) atom bonded to three oxygen (O) atoms, with an overall -2 charge on the ion. The Lewis structure illustrates the distribution of electrons, including lone pairs and bonding pairs. 2.
This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons.The f-subshell holds 14 electrons and the g-subshell contains up to 18 electrons.Metals in the middle of the periodic table become more stable by emptying a shell, half-filling it, or completely .GTAinside is the ultimate GTA Mod DB and provides you more than 95,000 Mods for Grand Theft Auto: From Cars to Skins to Tools to Script Mods and more.
sulfate ion lewis structure|How to Draw the Lewis Structure for the Sulfate Ion